Types of bacteriological research. Guidelines. Methods of bacteriological study of opportunistic microorganisms in clinical microbiology
APPROVED by the Ministry of Health of the RSFSR on December 19, 1991
Guidelines compiled by A.N. Kalyuk.
Bacteriological studies for opportunistic pathogens
A comprehensive laboratory study of microflora includes bacterioscopic and bacteriological studies of the material, carried out in dynamics upon admission to inpatient treatment, during treatment, as well as according to indications in patients treated on an outpatient basis. It is advisable to inoculate the diagnostic material on dense nutrient media, which excludes the suppression of the growth of one microorganism by another and allows a quantitative estimate of the number of grown colonies. The intensity of growth of microorganisms can be expressed in crosses and correspond to the content of a certain number of microbial cells in 1 ml of diagnostic material:
++++ Abundant growth of confluent colonies (10 m/cl)
+++ massive growth of isolated colonies (10 m/cell)
++ moderate growth of many countable colonies (at least 50) (10-10 m/kl)
+ poor growth of single colonies (30-50) (10 m/cell).
With dosed sowing, the absolute content of microorganisms in 1 ml or 1 g of the test material is determined. The etiologically significant content of bacteria in 1 ml (1 g) of the material is 10 and above. The quantitative predominance of a certain type of microorganism is one of the indicators of its participation in the pyoinflammatory process. The final interpretation of the results of bacteriological examination is made after studying the anamnestic data, clinical symptoms, and the results of antibiotic therapy. When sending material for sowing, certain rules must be observed. The material should be examined before the start of antibiotic therapy or after such a period after the administration of antibacterial drugs that is necessary for their elimination from the patient's body (2-3 days for sputum tests, 4-7 days for urine). The use of antibiotics reduces the frequency of isolation of microorganisms by 3-4 times. Crops of diagnostic material are carried out in dynamics (3-5 times), which clarifies the etiology of the disease, makes it possible to trace the duration of the persistence of the pathogen, to control the effectiveness of the therapy. The interval between collection and sowing of the material should not exceed 1-2 hours.
The study of the microflora of the upper respiratory tract(pharynx, nose, mouth). The material for the study is: mucus, purulent discharge, crusts, film, pieces of infiltrates during biopsy. Material for microbiological research from oral cavity taken on an empty stomach with a sterile cotton swab from the mucous membrane at the exit of the ducts of the salivary glands, the surface of the tongue, from sores (scraping with a spoon), from the most affected places. If there is a film, the latter is removed with tweezers. Material from the nasal cavity is taken with a dry sterile cotton swab. The material is seeded on Petri dishes with blood, yolk-salt agars, Saburo's medium. When sowing with a swab, the material is rubbed into the medium from the entire surface of the swab in a small area of 1-2 cm, and then with strokes over the entire surface. Simultaneously with sowing, smears are prepared and stained according to Gram.
Study of the microflora of the lower respiratory tract. The main material for the study is sputum, which is collected on the day of the study, in the morning, after brushing your teeth and rinsing your mouth with freshly boiled water. At copious excretion sputum, the first portions should be coughed up into a spit, and the subsequent ones are collected in sterile dishes and delivered to the laboratory. To study the microflora of sputum, both sowing of undiluted sputum (qualitative method) and the dilution method, which is called quantitative, are used. With a qualitative method for sowing, purulent lumps of sputum are used, washed in saline from the microflora of the oral cavity. With quantitative methods, 1 ml of sputum is homogenized. Then dilutions are made to reduce the number of oral microorganisms in it. With both methods, a smear is prepared simultaneously with the culture, which is stained by Gram. Purulent and mucopurulent sputum, in which there are leukocytes and cells of the alveolar epithelium, cells interspersed in mucin, the presence of which is characteristic of the excretion of the lower respiratory tract, are subject to research. Pay attention to the microflora prevailing in the smear of native sputum, especially capsular diplococci (pneumococci), small gram-negative rods (Pfeiffer rod), etc.
quality method. In the laboratory, sputum is poured into a Petri dish, 2-3 purulent lumps are selected, which are washed once in saline, and then inoculated on blood and yolk-salt agars, Endo and Saburo media. Sowing is carried out with a sterile glass spatula, evenly rubbing the material on the surface of the nutrient medium. Disks with antibiotics (streptomycin, penicillin, tetracycline, erythromycin, and levomycetin) are placed on the blood agar plate immediately after inoculation, which makes it possible to obtain express information on the drug sensitivity of the microflora prevailing in the inoculation. On the second day, the number of grown colonies is taken into account (the growth of more than 50 colonies is considered etiologically significant), the homogeneity of the population and drug sensitivity during their growth in a monoculture.
quantitative method. 1 ml is taken from the sputum delivered to the laboratory, 9 ml of meat-peptone broth is added and homogenized in a jar with beads for 20 minutes. Ten-fold serial dilutions are prepared from the resulting emulsion. Sowing is carried out in the reverse order from a smaller dilution. Inoculate 0.1 ml of diluted sputum 10 and 10 per blood agar plate. Sowing on yolk-salt agar, Endo and Sabouraud media are made from the initial dilution of 1:10. Crops are incubated for a day at 37°C. On the second day, the cups are viewed and the number of each of the types of microorganisms in millions is taken into account. A bacterial content of 10 m / cl and above in 1 ml of sputum is recognized as diagnostically significant.
Sowing of bronchial washings, lavage fluid. Lumps of mucus are taken from the test material, which, without prior washing in physiological saline, are inoculated on solid nutrient media (see sputum culture) and in a test tube with sugar broth. In the absence of lumps of mucus, the material collected in a Pasteur pipette is inoculated. Incubation during the day at 37°C.
Study of the microflora of the eyes. Samples for research are taken by a doctor with a sterile cotton swab or glass rod. The material is taken from the affected areas and sown in 0.5% sugar broth. In the absence of growth, a negative response is issued after 48 hours.
Examination of swabs from the ear. The material is taken with a sterile cotton swab from the auditory canal and inoculated on blood and yolk-salt agar, Sabouraud medium, rubbing the material on the area of the medium, and then rubbing it over the entire cup.
Urine study. The study is subject to the average portion of morning urine obtained during normal urination or taken by a catheter. An indicator of bacteriuria, having clinical significance, the presence of 100,000 or more microbes in 1 ml of urine is considered.
First day of research. One standard (3 mm) bacteriological loop of urine (thoroughly mixed) is sown in sectors A, I, II and III in a Petri dish with 5% blood or simple agar. At the same time, in the area of the medium of sector A, sowing is done, evenly rubbing the material over the entire surface, then without taking new material, the same loop is done with strokes on the nutrient medium in sector I (3-4 strokes), from sector I to II, from II sectors - in III.
Table 1
The number of bacterial colonies in different sectors of the Petri dish, depending on the degree of bacteriuria (according to V.S. Rabinovsky and V.V. Rodoman)
|
The number of bacteria in 1 ml of urine |
The number of colonies in different sectors of the Petri dish |
|||
|
Less than 1 thousand |
no growth |
no growth |
||
|
very big |
||||
|
from unit up to 25 |
||||
Second day of research. The degree of bacteriuria is determined according to Table 1, depending on the sector in which the growth of colonies of the microorganism is found. In the presence of less than 100 thousand microbes in 1 ml of urine, the growth of colonies is observed only in sector A of the Petri dish. The appearance of colony growth in sector I indicates more a high degree bacteriuria. Counting colonies in the sector with the smallest growth is not difficult. The method of sector crops in most cases makes it possible to isolate the causative agent of the disease in pure culture already on the second day of the study.
Study of the microflora of wounds, punctates, exudates, resected tissues. Exudates and punctates are inoculated with a Pasteur pipette into test tubes with blood and simple meat-peptone agar, sugar broth. A swab with diagnostic material is sown on Petri dishes with 5% blood and 10% yolk-salt agars. The material is rubbed along the edge of the medium, and then spread over the dish using the same swab or bacteriological loop.
Study of the microflora of the female genital organs. The secretions are collected with a sterile cotton swab and inoculated on Petri dishes with 5% blood agar, yolk-salt agar and in a test tube with sugar broth, as well as on Endo medium.
Study of bile. Bile is collected during probing or during surgery in sterile tubes and delivered to the laboratory no later than 2 hours from the moment of sampling. 0.1 ml of bile is plated on a blood agar plate and Endo medium. Crops and the remaining source material is placed in a thermostat at 37°C. After 24 hours, the results of the primary inoculations are taken into account with the counting of the number of colonies of each species on dense nutrient media.
Blood study. Blood is sown at the bedside of the patient after careful treatment of the skin (alcohol, ether). From the cubital vein, 10 ml of blood is taken, which is poured into two flasks: the first with 150-200 ml of sugar broth and the second with thioglycol medium (5 ml each). Crops are kept in a thermostat for 10 days. On the 2nd, 3rd, 5th and 10th days, control seedings are made on Petri dishes with 5% blood agar. Sowing 5 ml of blood can be done in a vial with a nutrient medium in two phases: solid and liquid (bevel of 5% blood agar with 1% glucose and 50 ml of 0.5% sugar broth). This technique eliminates the need for multiple re-sowing, eliminates the possibility of contamination of the crop with the microflora of the environment, and allows you to take into account the number of grown colonies (i.e., to assess the intensity of bacteremia). Sowing is placed in a thermostat at 37°C for 10 days. The contents of the vials are shaken daily, and the slope of the vial is used to moisten the bevel surface of the dense nutrient medium. When colonies grow on the blood agar slant, smears are prepared from them and further identified according to the rules generally accepted in bacteriology. In the absence of growth of microorganisms on the 10th day, the final answer is given - the blood culture is sterile.
Research on intestinal dysbiosis. An arbitrary amount of feces is taken on pre-prepared and weighed (subparchment or wax paper) sterile papers sized 3x2 and weighed on a torsion scale. The paper together with the material is placed in a sterile test tube. The weight of the sample of feces, minus the weight of the paper, is multiplied by 9. The sum obtained after multiplication is equal to the amount of saline that must be added to the test tube. Dilution 1:10 (I).
For example: the weight of a piece of paper is 20 mg
weight of faeces with a piece of paper 420 mg
420-20=400 mg; 400 mg9=3600 (3.5 ml).
After emulsification with a glass rod or a sterile pipette, the suspensions are allowed to stand at room temperature for 10-15 minutes and 0.1 ml is transferred to the next test tube with 9.9 ml of saline (dilution 10). Then the feces are diluted to a titer of 10. From the main dilution (10), inoculation is carried out on solid nutrient media to isolate pathogenic microbes of the intestinal family (Ploskirev, Levin medium). At the same time, a massive (0.5-1.0) inoculation is made on liquid enrichment media (Muller, selenite, magnesium). From a test tube in which the faeces are diluted to 10, 0.1 ml are added to the surface of the Sabouraud and JSA medium. From a dilution of 10, inoculations are made on plates with 0.5% blood agar and Endo medium, 0.1 ml each. To obtain the growth of isolated colonies, glass beads or spatulas are used. Glass round beads 12-14 pieces (sterilized in advance) are lowered into a cup with seed. With slight rocking of the cup with beads for 1 min, the material is evenly distributed over the nutrient medium. Sowing with beads starts from the medium on which the largest dilution was sown (10), transferring the beads to a smaller dilution. To isolate anaerobic bifidobacteria, inoculate from dilutions of 10, 10 and 10 into 2 tubes (0.1 and 1 ml each) of Blaurock's medium regenerated for 1 hour. After inoculation, the tubes are vigorously rotated between the palms to evenly distribute the suspension. Environment for growing aerobes is placed in a thermostat at 37°C (Sabouraud - at 20°) for 18-24 hours. The growth of anaerobes on Blaurock's medium is taken into account after 48-72 hours. The next day after sowing, the number of Escherichia coli and other microbes in 1 g of feces is determined by the number of colonies that have grown on the appropriate nutrient medium, recalculated for the amount of inoculated material and the degree of its dilution. So, if 30 lactose-negative colonies grew on Endo medium when 0.1 ml of feces was inoculated from a dilution of 10 (1: 100,000), the calculation should be multiplied by 30 by 10 and by 100,000, i.e. in 1 g there will be 30,000,000 lactose-negative enterobacteria. The number of lactose-negative and hemolytic colonies of Escherichia coli, the presence of staphylococcus, Proteus and other microorganisms are taken into account. The enzymatic properties and drug sensitivity of microorganisms are determined. Smears are prepared from test tubes with Blaurock's medium. Under the microscope, bifidobacteria have the appearance of characteristic gram-positive rods, thickened or branched at the ends, arranged in the form of a Roman numeral V, often in the form of clusters. The answer of the bacteriologist indicates the percentage or absolute number of each group of microorganisms.
Identification of microorganisms. Methods for identifying microorganisms are based on the study of morphological, cultural, biochemical, antigenic, and other properties of cultures.
Morphological properties are studied by bacterioscopy of diagnostic material and smears from colonies grown on solid and liquid culture media. Smears on glass slides are fixed on a burner flame or in liquid fixatives (96 °, alcohol, Nikiforov's mixture) stained according to Gram. When viewing smears from sputum, all available microflora is assessed: the presence of clusters of gram-positive cocci (staphylococci, micrococci), chains of gram-positive cocci (streptococci), small lanceolate diplococci surrounded by a zone of unstained capsule (pneumococcus), gram-negative cocci (neisseria); Gram-negative rods (intestinal, Pseudomonas aeruginosa, Proteus); Gram-negative rods with rounded ends surrounded by a capsule in the form of a light halo (Klebsiella), small Gram-negative rods in the form of clusters (hemophilic bacteria), etc. Bacterioscopic examination is indicative. Further research includes inoculation of the material on nutrient media, isolation of pure cultures, their identification and determination of drug susceptibility. Cultural properties are studied when viewing grown cultures on solid and liquid nutrient media. On dense media, the size of colonies, color, transparency, shape, presence of pigment, hemolysis around the colony and its nature, etc. are taken into account. On liquid media, their transparency, the presence of sediment (bottom growth) or a film on the surface of the medium are noted. The study of biochemical properties is based on the determination of enzymatic saccharolytic activity, the ability to utilize nutrients under aerobic and anaerobic cultivation conditions. The antigenic properties of cultures are studied by the interaction of bacteria and their antigens with the corresponding antisera (agglutination reactions, immunofluorescence, etc.). After studying the morphological and cultural properties, differential tests are carried out with pure cultures of microorganisms.
Gram-positive cocci. Gram-positive cocci belong to the family Micrococcaceae, which includes the genus Micrococcus and Staphylococcus and the family Streptococcaceae.
Family Micrococcaceae. For medical microbiology, it is necessary to differentiate staphylococci from micrococci. They study morphological properties, hemolysis, the ability to grow on a medium with salt, pigment formation, fermentation of glucose to acid under anaerobic conditions, fermentation of glycerol. Micrococci have a 2-3 times larger cell size (0.5-3.5 µm), do not ferment glucose under anaerobic conditions and glycerol, and have a yellow to pink pigment. Differentiation of various types of staphylococcus is carried out according to a set of tests: plasma-coagulating ability, lecithinase activity, mannitol fermentation under anaerobic conditions, pigment formation, sensitivity to novobiocin (the test is positive for St. aureus and St. epidermidis and negative for St. saprophyticus). To isolate staphylococcus, the test material is inoculated on a differential diagnostic medium: yolk-salt agar. When stained by Gram, staphylococcus is stained gram-positive and is located singly, in pairs, or forms clusters in the form of irregular piles. Staphylococcus is resistant to elevated concentrations in sodium chloride (7-10%), which is used to isolate it from pathological material. When growing on meat-peptone broth, it causes its uniform turbidity and gives a flocculent precipitate. On dense nutrient media, staphylococcus grows in the form of round shiny colonies with smooth edges (0.5-1.5 mm in diameter). On the second day of the study, the quantitative growth of the grown colonies is assessed, lecithinase activity is taken into account, and a pure culture of the microbe is isolated (transplanted into tubes with milk or simple slant agar). On the third day - they put tests for differentiation and drug sensitivity.
When determining coagulase activity, lyophilized rabbit blood plasma is used, diluted with sterile saline 1:5 and poured into sterile tubes of 0.5 ml each. 1 loop of a daily agar culture of the test strain is inoculated into a test tube and placed in a thermostat at 37°C. Results are recorded after 30 minutes, 1 hour, 2 hours and 24 hours. All degrees of plasma coagulation from a small clot that remains motionless when the tube is inverted are considered positive.
Lecithinase activity is determined on yolk-salt agar. The reaction is recorded after 24-48 hours macroscopically by the presence of a cloudy zone and an iridescent corolla around the staphylococcus colonies, which indicates the presence of the lecithinase enzyme in them.
When studying the fermentation of mannitol, inoculation of a daily agar culture of the test strain is carried out with a corner in a column of 1% agar with mannitol and vaseline oil. During the fermentation of mannitol, the agar column turns blue. A positive reaction is considered when fermenting 2/3 of the agar column.
To determine the pigment formation of staphylococcus cultures, they are inoculated on 10% milk agar. Accounting in 18-20 hours.
Determination of the hemolytic ability of the culture of staphylococcus is carried out on 5% blood agar (donor blood without the addition of antiseptics) by the presence of enlightenment around the grown colonies, which are clearly detected in transmitted light. A positive hemolytic test on human blood agar is usually due to hemotoxins, while the main role in the pathogenesis of staphylococcal infections is played by alpha-toxin, which can be detected on rabbit blood agar.
Family Streptococcaceae. Streptococci are a large and rather heterogeneous group of microorganisms. The most studied are aerobic representatives: Streptococcus pyogenes, S. faecalis, S. pneumoniae. The microbe has a spherical shape, Gram-positive, in smears from dense nutrient media it is located in the form of short chains of 2-3 cocci, on liquid nutrient media it gives longer chains. When growing streptococci, one should take into account their increased need for nutrients Oh. Therefore, for the cultivation of streptococcus, nutrient media containing glucose (1%), blood (5-10%), serum (10-20%) are used.
ACTIVITY #4
TOPIC: PHYSIOLOGY OF MICROORGANISMS. BACTERIOLOGICAL (CULTURAL) RESEARCH METHOD. BIOCHEMICAL PROPERTIES OF MICROORGANISMS.
CHECKLIST
Nutrition of bacteria. Nutrients are sources of carbon and nitrogen. Classification of bacteria by types of nutrition Autotrophs and chemoorganotrophs
Growth factors and their sources. Sources of mineral elements.
Ways and mechanisms of transfer of nutrients through the membrane.
Energy requirements of bacteria. Ways of obtaining energy in autotrophs (photosynthesis, chemosynthesis). Sources and ways of obtaining energy in chemoorganotrophs.
Aerobic and anaerobic types of biological oxidation in bacteria. Aerobic, anaerobic, facultative anaerobic and microaerophilic bacteria. Ways to create anaerobic conditions.
Tasks, stages, advantages and disadvantages of the bacteriological (cultural) research method.
Growth and reproduction of microorganisms. Reproduction methods. Binary (simple) fission, mechanism. Reproduction of bacterial populations.
Principles and methods of cultivation of bacteria. The nutritional needs of microbes.
Nutrient media for the cultivation of bacteria. nutrient requirements. Classification of nutrient media.
Conditions and techniques for cultivating bacteria. Technique of sowing on nutrient media. Patterns and character of bacterial growth on solid and liquid nutrient media.
Methods for isolating pure cultures of aerobic and anaerobic bacteria.
Properties used to identify isolated crops.
INDEPENDENT AND LABORATORY WORK
Bacteriological method(stages):
1 1st stage isolation of a pure culture of aerobic bacteria: A) Microscopy of pathological material.
Gram staining of smears from pathological material. Drug sketch.
B) Mastering, under the guidance of a teacher, the technique of sowing pathological material with a bacteriological loop and a spatula on plate nutrient media.Inoculation of pathological material with a bacteriological loop on lamellar meat-peptone agar (MPA) to obtain isolated colonies.
Classification of culture media(specify areas of application)
1. By consistency:liquid (meat-peptone broth, bile, sugar broth), dense (2-3% agar) and semi-liquid (0.15-0.7% agar) media.
2. By origin:natural - from milk, meat. eggs, potatoes, human blood serum, animal and other products; artificial - 1) natural balanced mixtures of nutrients in concentrations and combinations necessary for the growth and reproduction of microorganisms, a universal source of nitrogen and carbon - peptones - products of incomplete breakdown of proteins using pepsin or various hydrolysates (fish, casein, yeast, etc.) .2) synthetic caccurate chemical composition Soton for mycobacteria, 199 for cells.
3. In composition: simple culture media (meat-peptone broth-MPB, meat-peptone agar-MPA) and with false (KA = MPA + 5-10% of animal blood)
4. By appointment:
BUT) general purpose - universal, intended for cultivation of any microorganisms (MPA,KA)
B ) Specialfor growing microorganisms that do not grow on universal media, differentiation of species and selective isolation of certain types of microorganisms:
elective (selective) to isolate certain types of microorganisms and suppress the growth of related ones - (salt agar for staphylococci).
differential diagnostic (DDS)-environments that make it possible to distinguish between types of bacteria by enzymatic activity; They With possess: 1) universal nutrient medium (MPA, KA); 2) differentiating factor - a chemical substrate (for example, carbohydrate), a different relationship to which is a diagnostic feature for a given microbe. 3) An indicator whose color change indicates a biochemical reaction. (environments of Endo, Ploskirev, Giss and others).
differential selective (DS) - environments that allow allocate bacteria of a certain species according to their physiological characteristics and differentiate from other species according to enzymatic activity They contain: 1) MPA 2) elective a chemical substrate that inhibits the growth of other types of bacteria . 3) differentiating factor - substrate to which is a diagnostic feature for this microbe;) 4.) An indicator whose color change indicates a biochemical reaction. (Wednesdays for staphylococci, ICA for salmonella, Ploskirev for shigella and salmonella).
B) Enrichment media for the reproduction and accumulation of bacteria of a certain type in clinical material (blood in 20% bile broth = salmonella, throat discharge in 10% serum + 2% tellurite = corynebacteria.)
D) Transport medium for the collection and delivery (preservation) of clinical material = 48 hours (Amies medium - semi-liquid agar + activated charcoal).)
Nutrient media(examples):
Wednesday Endo Medium type differential diagnostic for enterobacteria Nutrient Base MPA differentiating factor lactose 1% Indicator basic fuchsin decolorized with sodium sulfite. E.s oli decompose lactose to acid - colonies are red with a metallic sheen, pathogenic colorless;
Salt agar Medium type selective for isolation of staphylococci Nutrient Base MPA elective factor sodium chloride 10%
Wednesday Ploskirev Medium type differential selectivefor enterobacteria
Nutrient Base MPA elective factor bile salts differentiating factor lactose
Indicator neutral red
Yolk-salt agar Medium type differential selective for S . aureus _
Nutrient Base MPA elective factor sodium chloride 10%
differentiating factor egg yolk
Indicator No
2 Stage 2 bacteriological research method (isolation of pure culture):
A) The study of isolated colonies (escherichia, staphylococcus) on lamellar MPA.
|
Studied cultural properties |
1 type of colonies |
2 type of colony |
|
colony shape |
Regular shape, round |
Correct form |
|
Consistency |
homogeneous |
homogeneous |
|
Colony size |
medium (size 2-4 mm) | |
|
The nature of the edge |
with smooth edges |
with smooth edges |
|
Surface nature |
convex |
B) Preparation of smears from selected colonies (Gram stain).
C) Transfer of isolated colonies to slanted MPA for the accumulation of pure culture.
3 Isolation of a pure culture of anaerobic bacteria: inoculation of a soil suspension on the Kitta-Tarozzi medium to isolate pathogenic clostridia
Kitt-Tarozzi medium consists of nutrient broth, 0.5% glucose, and pieces of liver or minced meat to absorb oxygen from the medium. Before sowing, the medium is heated in a boiling water bath for 20-30 minutes to remove air from the medium. After sowing, the nutrient medium is immediately filled with a layer paraffin
Methods for creating anaerobiosis:
1.Physical- pumping out air, introducing a special oxygen-free gas mixture (usually N 2 - 85% CO 2 - 10%, H 2 - 5%), preliminary boiling of nutrient media, inoculation in a deep column of agar, filling the media with vaseline oil to reduce oxygen access, the use of hermetically sealed vials and test tubes, syringes and laboratory glassware with an inert gas, the use of tightly closed desiccators with a burning candle
2. Chemical- chemical oxygen scavengers are used.
3. Biological - joint cultivation of strict aerobes and anaerobes (aerobes absorb oxygen and create conditions for the reproduction of anaerobes - the Fortner method).
Wednesday Kitt - Tarozzi consists of a nutrient broth, 0.5% glucose and pieces of liver or minced meat to absorb oxygen from the environment. Before sowing, the medium is heated in a boiling water bath for 20-30 minutes to remove air from the medium. After sowing, the nutrient medium is immediately filled with a layerparaffin or vaseline oil to isolate from oxygen access.
4. Mixed - use several different approaches.
Special devices are used to create anaerobic conditions - anaerostats. Currently the simplest and most efficient equipment for creating anaerobic and microaerophilic conditions is a chemical method with special bags acting on the principle of absorbing atmospheric oxygen in hermetically sealed containers .
Wilson-Blair medium (tubes, cups):
Nutrient Base MPA Respiratory substrate glucose
Reducing factor sodium sulfite and ferric chloride sodium sulfiteNa 2 SO 3 → Na 2 S
For the Wilson-Blair environment, the base is agar with addition glucose , Clostridia form on this medium colonies black color due to restoration sulfite before sulfide - anion , which is connected with cations gland (II) gives black salt. Typically black on this education medium colonies , appear in the depth of the agar column .
Thioglycol medium (medium for sterility control): (tubes):
Nutrient Base BCH Respiratory substrate glucose Reducing factor sodium thioglycolate
Indicator resazurin
Zeissler blood glucose agar: (cups): Nutrient Base MPA, blood
Respiratory substrate glucose Reducing factor hemoglobin
The term "anaerobes" was introducedLouis Pasteurwho discovered in 1861bacteriabutyric fermentation.
BUTLecture 3 Physiology of microorganisms. Metabolism of bacteria .
The physiology of microorganisms includes :
types of food;
types of breathing;
cultivation (conditions, environments, character and growth rate);
biochemical activity;
variability;
isolation biologically active substances, toxins and other pathogenicity factors;
sensitivity to antibiotics, bacteriophages, bacteriocins;
other biological properties.
Metabolism of bacteria - a set of physical and chemical processes (chemical transformations and reactions) aimed at reproducing structures and ensuring the vital functions of a microbial cell, such as:
growth and reproduction;
deposition of reserve food material;
transport of nutrients into the microbial cell;
release of metabolic products (toxins, enzymes, antibiotics and other biologically active substances);
motion;
spore formation;
adhesion on sensitive receptors of host cells and penetration into them;
various adaptive responses to changes in the external environment.
Anabolism- a set of biochemical reactions that carry out the synthesis of cell components.
catabolism- a set of reactions that provide the cell with energy.
Metabolism study scheme - stages:
1. Initial (peripheral) metabolism - the penetration of substances into the cell from the outside and decay to intermediate products.
2. Amphibolism (intermediate metabolism) - the formation of intermediate metabolic products common to catabolic and anabolic pathways.
3. The final, strictly specialized stages of constructive metabolism (lead to the construction of cell structures) and energy metabolism (ATP formation).
Mechanisms for the penetration of nutrients into the cell:
Simple diffusion (for true solutions). energy independent process.
Facilitated diffusion ("steam downstream") - in the direction of the concentration gradient with the participation of carrier proteins. energy dependent process.
Active transport is against the concentration and electrochemical gradient with the participation of permeases (amino-, hydroxy-acid, ionic, etc.). The process goes with the expenditure of ATP energy, depends on the charge of substances and their transformation in the process of transfer.
Microorganisms are divided into two groups according to their ability to absorb carbon sources: autotrophs (lat. autos - myself, trophy - nutrition) synthesize all the carbon-containing components of the cell from CO 2 as the only source of carbon and heterotrophs (lat. heteros - the other, “feeding at the expense of others”) use a variety of organic carbon-containing compounds.
Depending on energy sources, microorganisms are also divided into phototrophs (photosynthetic), capable of using solar energy, and chemotrophs (chemosynthetic), receiving energy through redox reactions.
Depending on the electron donors used, bacteria are divided into lithotrophs (using inorganic electron donors) and organotrophs (using organic compounds).
Prototrophs- microorganisms capable of synthesizing all the organic compounds they need from glucose and ammonium salts.
Auxotrophs- microorganisms incapable of synthesizing any organic compounds. They obtain these compounds in finished form from the environment or the human body.
Enzymes(from the Greek. fermentum-sourdough) - highly specific protein catalysts present in all living cells, without which life and reproduction are not possible. Enzymes recognize their respective metabolites (substrates), interact with them, and accelerate chemical reactions. Enzymes are proteins.
The enzyme composition of a microorganism is determined by the genome and is a fairly stable trait. The determination of enzymes is widely used for the biochemical identification of bacteria.
Endoenzymes catalyze metabolism within the cell.
Exoenzymes are secreted by the cell into the environment.
Constitutive enzymes are constantly synthesized at certain concentrations.
inducible enzymes are enzymes whose concentration increases with the intake of the corresponding substrate.
Enzymes of aggression: hyaluronidase, fibrinolysin, neuraminidase, collagenase, lecithinase (licitovitellase), coagulase, urease, amino acid decarboxylases, deoxyribonuclease.
cultivation- obtaining cultures of microorganisms in an artificial nutrient medium.
Cultivation goals:
obtaining pure cultures of pathogenic microorganisms and their identification;
accumulation of biomass of BAS producers (vitamins, hormones, amino acids, antibiotics, etc.);
obtaining diagnostic and prophylactic preparations (vaccines, diagnosticums);
storage of reference museum cultures;
in sanitary microbiology to determine sanitary-indicative microorganisms - indicators of environmental pollution.
culture- a population of microorganisms grown on a nutrient medium.
pure culture- a population of one type of microorganisms grown from an isolated colony on a nutrient medium.
Most pathogenic microbes are grown on nutrient media at 37°C for 1-2 days.
Classification of culture media
By consistency: liquid, semi-liquid, dense.
Origin: natural (milk, potatoes), artificial, semi-synthetic, synthetic
In composition: simple (MPA, MPB, vegetables, milk), complex (1% glucose, 10-20% serum, 20-30% ascitic fluid, 5-10% defibrinated blood).
By appointment:
universal - media on which many types of bacteria grow well. These include meat-peptone broth (MPB) and meat-peptone agar (MPA);
special - media specially prepared to obtain the growth of bacteria that do not grow on universal media;
differential diagnostic - environments that make it possible to distinguish one type of bacteria from others by enzymatic activity;
selective - media containing substances used by microorganisms of certain species and preventing the growth of other microorganisms. Selective media allow you to select certain types of bacteria from the material under study;
differential-selective - environments that combine the properties of differential diagnostic and selective environments;
preservative;
concentrating.
Reproduction of bacteria on liquid and dense nutrient media.
Growth coordinated reproduction of all components of a bacterial cell and an increase in its biomass. reproduction- reproduction and increase in the number of cells, leading to the formation of a bacterial population.
Bacteria are characterized by a high rate of reproduction. The reproduction rate depends on the species, the composition of the nutrient medium, pH, temperature, and aeration.
On dense nutrient media, bacteria form clusters of cells called colonies. Colonies different types differ in size, shape, consistency, color, nature of the edges, nature of the surface, transparency.
The nature of growth on liquid nutrient media: filmy (formation of a film on the surface of the nutrient medium), diffuse turbidity, near-bottom (precipitation).
Phases of development of a bacterial population
Initial stationary phase (~ 1-2 hours). The number of bacteria does not increase, the cells do not grow.
Lag phase or breeding delay phase (~ 2 hours).
Log-phase - logarithmic or exponential phase (~ 3-5h). The population is divided maximum speed and there is an increase in individuals exponentially.
Phase of negative acceleration (~ 2 hours). Associated with the depletion of the limiting metabolite or the accumulation of toxic metabolic products.
Stationary phase of the maximum. The number of formed and dying cells is the same.
Phase of accelerated death (~ 3 hours).
Logarithmic death phase (~5).
The phase of decreasing the rate of death - the remaining living individuals go into a dormant state.
Energy metabolism of bacteria
Aerobes- microorganisms that use the aerobic (oxidative) type of biological oxidation of substrates. Metabolism of aerobes is carried out only in the presence of a high concentration of free oxygen in the habitat, which acts as the final acceptor of electrons taken from the substrate. The cultivation of aerobes is carried out on media with full access to atmospheric oxygen.
obligate anaerobes- microorganisms using the anaerobic type of biological oxidation (fermentation). Metabolism occurs only in environments with a low redox potential in the absence of oxygen.
An increase in the oxygen concentration in the environment leads to the death of vegetative forms.
The amount of energy extracted during fermentation is small, so obligate anaerobes are forced to ferment a large amount of substrate.
Facultative anaerobes- microorganisms capable of extracting energy from substrates by aerobic (oxidative) and anaerobic (fermentative) pathways of biological oxidation. Metabolism can be carried out both under conditions of full access of oxygen to the environment, and under conditions of anaerobiosis.
Methods for creating anaerobiosis
Physical
sowing in a column of sugar MPA;
boiling (regeneration) of liquid nutrient media followed by oil coating;
mechanical removal of oxygen in anaerostats;
replacement of oxygen by an indifferent gas;
Veillon-Vignal tubes.
Chemical
apparatus of Aristovsky;
Omelyansky's candle (alkaline solution of pyrogallol);
the use of chemical oxygen acceptors: glucose, pyruvic acid, sodium formic acid, etc.
Biological
Kitta-Tarozzi Wednesday
Fortner method
Anaerobes - organisms that obtain energy in the absence of access oxygen by substrate phosphorylation , the end products of incomplete oxidation of the substrate can be oxidized with more energy in the formATP in the presence of a terminal proton acceptor by organisms that .
Anaerobic respiration- aggregate biochemical reactions, occurring in the cells of living organisms when used as the final proton acceptor, does not oxygen, and other substances (for example, nitrates) and refers to the processes energy metabolism(catabolism,dissimilation), which are characterized oxidationcarbohydrates,lipids And amino acids to low molecular weight compounds.
BUT 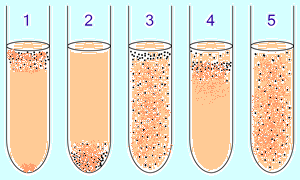 aerobic and anaerobic bacteria are preliminarily identified in a liquid nutrient medium by the O2 concentration gradient:
aerobic and anaerobic bacteria are preliminarily identified in a liquid nutrient medium by the O2 concentration gradient:
1. obligate aerobic(oxygen-demanding) bacteria mainly gather at the top of the tube to absorb the maximum amount of oxygen. (Exception: mycobacteria - film growth on the surface due to the wax-lipid membrane.)
2. obligate anaerobic bacteria gather at the bottom to avoid oxygen (or not grow). 3. Facultative bacteria are collected mainly in the upper ( oxidative phosphorylation is more beneficial than glycolysis), however, they can be found throughout the medium, since they do not depend on O 2. 4 . microaerophiles are collected in the upper part of the tube, but their optimum is a low concentration of oxygen. five. Aerotolerant anaerobes do not react to oxygen concentrations and are evenly distributed throughout the test tube.
D 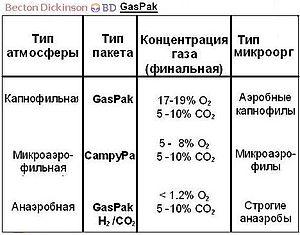 for measurement capacity environments M. Clark proposed to use the pH20 value - negative logarithmpartial pressure gaseous hydrogen. The range characterizes all degrees of saturation aqueous solution hydrogen and oxygen. Aerobes grow at a higher potential, facultative anaerobes, and obligate ones at the lowest.)
for measurement capacity environments M. Clark proposed to use the pH20 value - negative logarithmpartial pressure gaseous hydrogen. The range characterizes all degrees of saturation aqueous solution hydrogen and oxygen. Aerobes grow at a higher potential, facultative anaerobes, and obligate ones at the lowest.)
Classification of anaerobes, distinguish:
Facultative anaerobes
Capneistic anaerobes and microaerophiles
Aerotolerant anaerobes
Moderately strict anaerobes
obligate anaerobes
If an organism is able to switch from one metabolic pathway to another (for example, from anaerobic respiration to aerobic and vice versa), then it is conditionally referred to as facultative anaerobes .
Until 1991, a class of capneistic anaerobes was distinguished in microbiology, requiring a reduced concentration oxygen and increased concentration of carbon dioxide (Brucella bovine type - B. abortus)
The use of the bacteriological method makes it possible to isolate the pathogen in a pure culture from the material obtained from the patient, and to identify it based on the study of a complex of properties. Most bacteria are capable of cultivation on various artificial nutrient media (except for chlamydia and rickettsia), so the bacteriological method is important in the diagnosis of many infectious diseases.
If a positive result is obtained, the bacteriological method makes it possible to determine the sensitivity of the isolated pathogen to antimicrobial drugs. However, the effectiveness of this study depends on many parameters, in particular on collection conditions and his transportation to the laboratory.
TO basic requirements requirements for the selection and transportation of material for bacteriological examination include:
- taking material before the start of etiotropic treatment;
- observance of sterility conditions when collecting material;
- technical correctness of material collection;
- a sufficient amount of material;
- ensuring the temperature regime of storage and transportation of the material;
- reduction to the minimum time interval between the collection of material and sowing on dense nutrient media.
Transportation of the material to the laboratory should be carried out as soon as possible, but not more than within 1-2 hours after its taking. Samples of the material must be at a certain temperature; in particular, normally sterile materials (blood, cerebrospinal fluid) are stored and delivered to the laboratory at 37 °C. Non-sterile materials (urine, respiratory secretions, etc.) are stored at room temperature for no more than 1-2 hours or no more than a day at 4 °C (household refrigerator conditions). If it is impossible to deliver samples to the laboratory within the prescribed timeframe, it is recommended to use transport media designed to preserve the viability of pathogens under conservation conditions.
Blood for research should be taken from the patient during the rise in body temperature, at the beginning of the onset of fever. It is recommended to examine 3-4 blood samples taken with an interval of 4-6 hours, which is reasonable in terms of reducing the risk of "missing" transient bacteremia and increasing the ability to confirm the etiological role of opportunistic microflora isolated from the blood if this microflora is found in several samples of venous blood. A blood sample in the amount of 10 ml in an adult and 5 ml in children is inoculated into at least two vials with a medium for aerobic and anaerobic microorganisms in a ratio of 1:10. A single study of arterial blood is also desirable.
Take cerebrospinal fluid(CSJ) is produced by a doctor with a lumbar puncture in the amount of 1-2 ml in a dry sterile tube. The sample is immediately delivered to the laboratory, where its study is also started immediately. If this is not possible, the material is stored at 37 °C for several hours. Significantly increases the number of positive results of bacteriological examination by sowing 1-2 drops of CSF in a test tube containing a semi-liquid medium with glucose, and in a Petri dish with "blood" agar. To send the material, isothermal boxes, heating pads, thermoses or any other packaging where the temperature is maintained at about 37 ° C is used.
Excreta for bacteriological examination, they are taken with sterile wooden spatulas in the amount of 3-5 g into a sterile vessel with a tightly closed lid. The study of the taken material should be started no later than 2 hours later. If it is not possible to start the study during this time, a small amount of material should be taken and placed in an appropriate transport medium. When selecting feces, one should strive to send pathological impurities (mucus, pus, epithelial particles, etc.) for research, if any, avoiding the ingress of blood impurities with bactericidal properties into the material.
To take the material, rectal swabs (with a cotton tip) can be used. The swab should be moistened with sterile isotonic sodium chloride solution or transport medium (not oil gel). It is introduced per rectum to a depth of 5-6 cm and, turning the tampon, carefully remove it, controlling the appearance of fecal color on the tampon. The swab is placed in a dry test tube if the study of the material is started within 2 hours, otherwise - in the transport medium.
Urine(an average portion of freely released urine) in the amount of 3-5 ml is collected in a sterile dish after a thorough toilet of the external genital organs. It is preferable to take morning portions of urine.
Bile collected during duodenal sounding in treatment room separately in portions A, B and C into three sterile test tubes, observing the rules of asepsis.
Wash water of the stomach collected in sterile jars in the amount of 20-50 ml. It should be borne in mind that gastric lavage in these cases is carried out only with indifferent (not having a bacteriostatic or bactericidal effect on microorganisms) solutions - preferably boiled water (without adding soda, potassium permanganate, etc.).
Sputum. Morning sputum released during a coughing fit is collected in a sterile jar. Before coughing, the patient brushes his teeth and rinses his mouth with boiled water in order to mechanically remove food debris, desquamated epithelium and microflora of the oral cavity.
Flushing water of the bronchi. During bronchoscopy, no more than 5 ml of isotonic sodium chloride solution is injected, followed by suction into a sterile tube.
Discharge of the pharynx, oral cavity and nose. The material from the oral cavity is taken on an empty stomach or 2 hours after eating with a sterile cotton swab or a spoon from the mucous membrane and its affected areas at the entrances of the ducts of the salivary glands, the surface of the tongue, from sores. If there is a film, the latter is removed with sterile tweezers. Material from the nasal cavity is taken with a dry sterile cotton swab, which is inserted deep into the nasal cavity. Material from the nasopharynx is taken with a sterile posterior pharyngeal cotton swab, which is carefully inserted through the nasal opening into the nasopharynx. If a cough begins at the same time, the swab is not removed until the cough ends. To conduct an analysis for diphtheria, films and mucus from the nose and pharynx are simultaneously examined, taking the material with different swabs.
The test material is inoculated on dense nutrient media using special techniques to obtain the growth of individual colonies of microorganisms, which are then sifted in order to isolate a pure culture of the pathogen.
Certain types of bacteria are isolated using elective (selective) media that retard the growth of foreign microorganisms or contain substances that stimulate the growth of certain pathogenic microbes.
Microorganisms isolated on nutrient media identify, i.e. determine their species or type affiliation. Recently, for identification in healthcare practice, microtest systems are used, which are panels with a set of differential diagnostic environments, which speeds up the study. Microtest systems are also used to determine the sensitivity of microorganisms to antimicrobial drugs by diluting an antibiotic in a liquid nutrient medium.
When evaluating the results of a bacteriological study, the doctor should take into account that a negative result does not always mean the absence of a pathogen and may be associated with the use of antimicrobial drugs, high blood microcidal activity, and technical errors. Detection of a pathogenic microbe in material from a patient out of connection with clinical picture possible in the case of convalescent, healthy or transient bacterial carriage.
Isolation from the blood, subject to all asepsis rules, of conditionally pathogenic microorganisms (Staphylococcus epidermidis, Escherichia coli) and even saprophytes should be considered a manifestation of bacteremia, especially if these microbes are found in more than one material sample or in different substrates (blood, urine), since when decrease in the immunoreactivity of the body, these and other "non-pathogenic" microorganisms can be the causative agents of infectious processes, including sepsis.
A certain difficulty is interpretation of the results of bacteriological examination of non-sterile media, namely the proof of the etiological role of opportunistic microorganisms. In this case, such indicators as the type of isolated cultures, the number of microbial cells of a given type in the material, their repeated isolation during the course of the disease, the presence of a monoculture or an association of a microorganism are taken into account in a complex.
Yushchuk N.D., Vengerov Yu.Ya.
Method of bacteriological research. Isolation of the pathogen from the blood (hemoculture) is an early method for diagnosing diseases. Bacteremia in patients with typhoid-paratyphoid disease appears at the end incubation period and does not disappear during the entire febrile period of the disease and during relapses. The results of bacteriological examination to a certain extent depend on the timing of the collection of material and the amount of blood inoculated. The earlier blood cultures are performed from the onset of the disease, the greater the likelihood of detecting the pathogen. In the first week of typhoid fever, the patient's blood is taken from the cubital vein in an amount of 10 ml, at a later date and during relapses - 20 ml.
On the first day of the study blood is inoculated in a ratio of 1:10 into one of the liquid media. The specified ratio must be strictly observed, since with a smaller dilution of blood, microbes can die due to its bactericidal action. For sowing, they use: 10% or 20% solution of bile broth, poured into 50-100 ml bottles, meat-peptone broth with the addition of 1% glucose, sterile distilled water - according to the method of N. N. Klodnitsky, in which erythrocyte lysis occurs, the products of their decay at the same time serve as a good nutrient medium for the reproduction of bacteria. You can also use sterile tap water. The best results are obtained by inoculating the material on bile broth. If it is not possible to perform a blood culture on the spot, the serum together with a clot or citrated blood (10 ml of blood is poured into a test tube with 2 ml of 5% sterile sodium citrate) is sent to the laboratory. The blood clot is crushed in the laboratory and inoculated into one of the listed media. The vials are placed in a thermostat at a temperature of 37 ° C.
Second day of research. After 14-24 hours from the start of the study, the material is inoculated into a Petri dish on Endo's medium or medium with eosin and methylene blue (Levin's medium). It is not recommended to use Ploskirev's medium for sowing, since typhoid-paratyphoid bacilli in the blood are obligate paratrophs by the type of nutrition and do not immediately change this type of nutrition to metatrophic (that is, dead organic substrates). Therefore, on this medium containing bile salts, typhoid fever bacilli grow very poorly or do not grow at all. In the absence of bacterial growth after the first sowing, the next ones are produced after 48, 72 hours and on the 5th and 10th days. If at the same time the pathogen could not be isolated, a negative answer is issued. In doubtful cases, it is recommended periodically - 1 time in 3-4 days - to produce crops up to the 24th day from the moment of taking the material. A negative answer is still issued on the 7th day.
Third day of research. “Suspicious” colonies grown on Endo and Levin media (colonies of pathogenic microbes on Endo’s medium are colorless) are identified, for which 2-3 colonies are subcultured onto slant agar and Ressel’s medium.
Fourth day of research. The results of inoculation on the Ressel medium are taken into account and recorded, the morphological properties of the isolated cultures are studied in a Gram-stained smear. Mobility is determined - the presence or absence of flagella - in a hanging or crushed drop taken from a 4-6-hour broth culture. To do this, agar culture (one loop) is inoculated in 1 ml of slightly warmed broth. The selected cultures (2-3 test tubes) are subcultured on Giss media with mannitol, sucrose and oblique agar, as well as in 2 test tubes with meat-peptone broth, in which filter paper strips moistened with special solutions for the determination of hydrogen sulfide and indole are placed under the stoppers (unfolded "variegated row").
Fifth day of research. Changes are registered on the expanded "variegated row". In the presence of gas formation, an agglutination reaction is performed with a mixture of salmonella sera. At positive reaction carry out an agglutination reaction with O- and H-sera and issue a final answer based on the totality of all signs.
Isolation of myeloculture is carried out by inoculation of the obtained bone marrow punctate in 3-5 ml of sterile bovine bile or in 25-30 ml of 10% bile broth, put it in a thermostat and the next day it is reseeded onto Endo or Wilson-Blair media. In the future, the stages of research are the same.
Isolation of bacteria from feces. From the 8th to the 10th day of illness, more often from the third week, in patients with typhoid fever, paratyphoid bacteria are excreted with feces. For research, the last portions of liquid feces are taken, emulsified in an isotonic solution of sodium chloride (at a ratio of 1:10) and left for 30 minutes until large particles settle. For sowing, a drop of material is taken from the surface of the liquid.
On the first day of the study, the material is inoculated on enrichment media - bile broth, magnesium media, Muller, Kaufman - and placed in a thermostat. On the second day of the study, from the enrichment medium, inoculation is done on plates with Ploskirev, Endo or Levin and Wilson-Blair media (bismuth-sulfite-agar). Subsequent stages of the study are the same as for the isolation of blood culture.
Isolation of bacteria of the typhoid-paratyphoid group from the urine is best done from the second to third week of the disease. Before taking the material, the external opening of the urethra should be washed with a sterile isotonic solution of sodium chloride; in women, it is better to take urine with a catheter. For research take 20-30 ml of urine. After centrifugation, the sediment is inoculated into 2 cups with Ploskirev's medium or bismuth-sulfite-agar. The supernatant is inoculated on an enrichment medium (10% bile broth) and placed in a thermostat for 24 hours, after which it is inoculated into 2 cups of one of the elective media. The isolated colonies are identified in the usual way.
Examination of duodenal contents - bile. The method is more often used in the stage of convalescence. Bile is collected during probing in sterile test tubes. The duodenal contents are inoculated into vials with 50 ml of broth, and the rest of the material, together with the inoculations, is placed in a thermostat at 37 ° C. The next day, inoculation is done in 2 cups with a dense differential medium. Selected colonies are identified by the described method.
Highly sensitive and promising in the early diagnosis of typhoid and paratyphoid fever is the method of immunofluorescence, which examines the blood from the first days of the disease, feces from the 10th day, duodenal contents on the 10th day normal temperature body. Typhoid-paratyphoid bacteria are marked with specific fluorescent sera, which are subsequently determined by fluorescent microscopy. The method allows diagnosing the disease in 10-12 hours from the start of the study.
Serological diagnosis of typhoid and paratyphoid diseases. From the second week of the disease, specific antibodies appear in the blood of patients, which can be determined using the Vidal reaction. In typhoid fever and paratyphoid, O- and then H-agglutinins accumulate. During the course of the disease, Vi-agglutinins are sometimes also detected, but the latter, unlike carriers, have no diagnostic value. The determination in the blood of patients of specific agglutinins to the causative agent of typhoid fever and paratyphoid fever (Vidal reaction) can help in establishing a diagnosis both in the acute period of the disease and during convalescence.
With salmonellosis, the Vidal reaction is an auxiliary diagnostic method. It should be remembered that often there are forms of the disease with a mild immunological response. Especially often low titers of agglutinins, up to their absence, are noted in patients treated with antibiotics.
For the Vidal reaction, 1-3 ml of blood is taken from a finger or cubital vein into a sterile test tube. In order to accelerate blood clotting, it is placed in a thermostat for 30 minutes. Clotted blood is circled with a glass pipette and placed in a refrigerator until a clear, settled serum appears. The clot is used for seeding (hemoculture). The agglutination reaction is put with H- and O-typhoid, A- and B-paratyphoid diagnosticums. The serum is diluted, starting with a titer of 1:100 to 1:800, according to the following method. 9.9 ml of a sterile isotonic sodium chloride solution and 0.1 ml of the test serum are poured into a test tube - a dilution of 1:100 is obtained. In 4 test tubes (according to the number of antigens used in the Vidal reaction), and in one serving as a serum control, 5 ml of diluted serum is poured into 1 ml. From the remaining 5 ml of serum (dilution 1:100), 1 ml is poured, and 4 ml of isotonic sodium chloride solution is added to 4 ml and a dilution of 1:200 is obtained. 4 ml from a dilution of 1:200 is also poured into 4 test tubes of 1 ml each, and 4 ml of isotonic sodium chloride solution is added again to the remaining 4 ml to obtain a dilution of 1:400. Subsequent dilutions (1:800, 1:1600) are produced in the manner described. In 4 test tubes, which are control antigens, pour 1 ml of isotonic sodium chloride solution. Into all experimental test tubes, except for those that serve as a serum control, 1-2 drops of the corresponding diagnosticums are poured. The rack with test tubes is shaken and placed in a thermostat at 37 ° C for 24 hours. H-agglutination (coarse-grained) occurs after 2 hours, O-agglutination (fine-grained) - much later. The intensity of the reaction is noted after 24 hours. The diagnostic titer of the Vidal reaction at a dilution of at least 1:200 in the presence of clinical manifestations.
It should be borne in mind that this reaction may be positive in other diseases - tuberculosis, malaria, brucellosis, malignant neoplasms and certain conditions (pregnancy). The Vidal reaction can also be positive in healthy individuals (domestic reaction), in those vaccinated and who have had a disease in the past (anamnestic reaction). To increase the specificity of the Vidal reaction, Fischer suggested diluting the serum with hypertonic sodium chloride solution (2.9 and 5.8%). This leads to the weakening or elimination of group reactions. The value of the agglutination reaction increases with repeated studies, when an increase in antibody titer is established with the dynamics of the disease. With paratyphoid A, the Vidal reaction may be negative or the titer of specific antibodies is less than the group.
In recent years, the reaction of passive hemagglutination (RPHA) with partial antigens of typhoid bacteria has been widely used to recognize the intestinal group of diseases. RPHA is cast with high sensitivity and specificity, it is positive from the 5th day of illness. The minimum diagnostic titer in patients with typhoid fever, paratyphoid fever, salmonellosis with O-antigen is 1:200. The reaction is put in dynamics to determine the increase in antibody titer.
Methods of laboratory diagnostics of bacteriocarrier in typhoid fever and paratyphoid fever. Bacteriological examination of feces, urine and duodenal contents is carried out according to generally accepted methods. The best results are obtained using selenite media.
Due to the frequency of isolation of bacteria, it is often not possible to sow the pathogen. In the vast majority of carriers of typhoid fever bacilli, microbes containing the Vi-antigen are found, and therefore Vi-antibodies appear in the blood of acute and chronic carriers (they are less common in the blood of patients). The diagnostic titer of the reaction is 1:20 and above. In parallel with the Vi-agglutination reaction (with warmed serum), the Vidal reaction (with native serum) is performed with H- and O-typhoid diagnosticums. In the sera of typhoid bacteria carriers, H-antibodies in titer from 1:200 to 1:800 are found in 60-80% of cases. The presence of a combination of H- and Vi-antibodies is of particular diagnostic value in identifying carriers of typhoid fever bacilli.
Of the additional research methods, a skin-allergic test with typhine, as well as RPHA with the Vi antigen, are used to identify the carriage of typhoid bacilli.
Thus, an important condition for the success of the fight against typhoid-paratyphoid diseases is the early and complete identification and neutralization of the source of infection. Currently, typhoid fever occurs sporadically. At the same time, the course of the disease is less long and is not accompanied by all the signs typical of the classical form, which makes clinical recognition difficult.
In connection with the above, a comprehensive laboratory examination is of great importance.
Bacteriological research method (BLMI)- a method based on the isolation of pure cultures of bacteria by cultivation on nutrient media and their identification to the species based on the study of morphological, cultural, biochemical, genetic, serological, biological, ecological characteristics of microorganisms.
Bacteriological diagnosis of infections is carried out using standard diagnostic schemes approved by the Ministry of Health.
Pure culture - bacteria of the same species, grown on a nutrient medium, the properties of which are in the process of being studied.
Strain- an identified pure culture of microorganisms of the same species, isolated from a specific source at a specific time. Strains of the same species may differ insignificantly in biochemical, genetic, serological, biological, and other properties, as well as in the place and time of isolation.
Goals of BLMI:
1. Etiological diagnosis: isolation of a pure culture of microorganisms and its identification.
2. Determination of additional properties, for example, the sensitivity of the microorganism to antibiotics and bacteriophages.
3. Determination of the number of microorganisms (important in the diagnosis of infections caused by UPM).
4. Typing of microorganisms, i.e., the determination of intraspecific differences based on the study genetic And epidemiological(fagovars and serovars) markers. This is used for epidemiological purposes, because it allows you to establish the commonality of microorganisms isolated from different patients and from different objects of the external environment, in different hospitals, geographical regions.
BLMI includes several stages, different for aerobes, facultative anaerobes and obligate anaerobes.
I. Stages of BLMI in the isolation of a pure culture of aerobes and facultative anaerobes.
Stage.
A. Collection, transportation, storage, Preliminary processing material. Sometimes, before sowing, selective processing of the material is carried out, taking into account the properties of the isolated microorganism. For example, before examining sputum or other material for the presence of acid-resistant Mycobacterium tuberculosis, the material is treated with acid or alkali solutions.
B. Seeding in enrichment medium(if necessary). It is carried out if the test material contains a small amount of bacteria, for example, when isolating a blood culture. To do this, blood taken at the height of fever in a large volume (8-10 ml in adults, 4-5 ml in children) is inoculated into the medium in a ratio of 1:10 (to overcome the action of blood bactericidal factors); sowing is incubated at a temperature of 37 0 C for 18-24 hours.
B. Microscopy of the test material. A smear is prepared from the test material, stained by Gram or other method and microscoped. Assess the present microflora, its quantity. In the course of further research, microorganisms present in the primary smear should be isolated.
G. Sowing on nutrient media in order to obtain isolated colonies. The material is inoculated with a loop or spatula by mechanical separation on a plate with a differential diagnostic or selective medium in order to obtain isolated colonies. After sowing, the dish is turned upside down (to avoid smearing the colonies with droplets of condensation liquid), signed and placed in a thermostat at a temperature of 37 0 C for 18-24 hours.
It should be remembered that when sowing and reseeding microbial cultures, the attention of the worker should be drawn to compliance with asepsis rules to prevent contamination of nutrient media and prevent infection of others and self-infection!
In the case of infections caused by opportunistic microorganisms, where the number of microorganisms present in the pathological material matters, a quantitative inoculation of the material is done, for which a series of 100-fold dilutions of the material (usually 3 dilutions) is prepared in a sterile isotonic sodium chloride solution in test tubes. After that, 50 μl of each dilution is sown on nutrient media in Petri dishes.
Stage.
A. Study of colony morphotypes on media, their microscopy. They look through the dishes and note the optimal nutrient medium, growth rate, and the nature of the growth of microorganisms. Choose to study isolated colonies located along the stroke, closer to the center. If several types of colonies grow, each is examined separately. Assess the signs of the colonies (table. 7). If necessary, the dishes with crops are viewed through a magnifying glass or using a microscope with a low magnification lens and a narrowed aperture. They study the tinctorial properties of different morphotypes of colonies; for this, a part of the colony under study is prepared smear, stained by Gram or other methods, microscopically and determine the morphology of the purity of the culture. If necessary, put indicative RA on glass with polyvalent serums.
B. Accumulation of pure culture. To accumulate a pure culture, isolated colonies of all morphotypes are subcultured into separate test tubes with slant agar or some other nutrient medium and incubated in a thermostat at +37 0 C (this temperature is optimal for most microorganisms, but it can be different, for example, for Campylobacterium spp.- +42 0 C, Candida spp. and Yersinia pestis- +25 0 C).
Kligler's medium is usually used as an accumulation medium for enterobacteria.
The composition of the Kligler medium: MPA, 0.1% glucose, 1% lactose, hydrogen sulfide reagent (iron sulfate + sodium thiosulfate + sodium sulfite), phenol red indicator. The initial color of the medium is raspberry-red, the medium is “slanted” in test tubes: it has a column (2/3) and a beveled surface (1/3).
Sowing in Kligler's medium is done by a stroke on the surface and an injection into a column.
Stage.
A. Accounting for growth on the accumulation medium, assessment of the purity of the culture in a Gram smear. growth patterns isolated pure culture. Visually clean culture is characterized by uniform growth. At microscopic examination a stained smear prepared from such a culture, morphologically and tinctorially homogeneous cells are found in it in different fields of view. However, in the case of pronounced pleomorphism inherent in some types of bacteria, cells with different morphology may occur simultaneously in smears from a pure culture.
If the Kligler indicator medium was used as the accumulation medium, then its color changes in the column and the beveled part are evaluated, according to which the biochemical properties are determined: the fermentation of glucose, lactose and the production of hydrogen sulfide. When lactose decomposes, the sloping part of the medium turns yellow; when glucose decomposes, the column turns yellow. With the formation of CO 2 during the decomposition of sugars, gas bubbles or a break in the column are formed. In the case of hydrogen sulfide production, blackening is noted along the injection due to the conversion of ferrous sulfate to ferrous sulfide.
The nature of the change in the color of the Kligler medium (Fig. 23) is explained by the unequal intensity of the breakdown of nitrogenous substances by microorganisms and the formation of alkaline products under aerobic (on a sloping surface) and anaerobic (in a column) conditions.
Under aerobic conditions, a more intense alkali formation occurs on a sloping surface than in a medium column. Therefore, during the decomposition of glucose present in the medium in a small amount, the acid formed on the beveled surface is quickly neutralized. At the same time, during the decomposition of lactose, which is present in a medium in high concentration, alkaline products are not able to neutralize the acid.
Under anaerobic conditions in the column, alkaline products are formed in an insignificant amount, so glucose fermentation is detected here.
Rice. 23. Kligler indicator medium:
1 - initial,
2 - with growth E. coli
3- with growth S. paratyphi B,
4 - with growth S. typhi
E. coli decompose glucose and lactose with gas formation, do not produce hydrogen sulfide. They cause yellowing of the column and beveled part with media breaks.
S. paratyphi decompose glucose with gas formation, lactose-negative. They cause yellowing of the column with breaks, the beveled part does not change color and remains raspberry. Wherein S. paratyphi B produce hydrogen sulfide (a black color appears during the injection), S. paratyphi A hydrogen sulfide is not produced.
S. typhi decompose glucose without gas formation, lactose-negative, produce hydrogen sulfide. They cause the column to turn yellow without breaks, the beveled part does not change color and remains raspberry, black color appears during the injection.
Shigella spp. glucose-positive, lactose-negative, do not produce hydrogen sulfide. They cause yellowing of the column (with or without breaks depending on the serovar), the beveled part does not change color and remains crimson.
B. Final identification of the pure culture(determination of the systematic position of the isolated microorganism to the level of species or variant) and determination of the sensitivity spectrum of the isolated culture to antibiotics.
To identify a pure culture at this stage, biochemical, genetic, serological and biological characteristics are studied (Table 8).
In routine laboratory practice, there is no need to study all properties during identification. Informative, accessible, simple tests are used, sufficient to determine the species (variant) affiliation of the isolated microorganism.
